- Colorectal Cancer Is Rising among Younger Adults. Some States Want to Boost Awareness.
- Rural Hospitals Built During Baby Boom Now Face Baby Bust
- Food Stamps Go Further in Rural Areas — Until You Add Transportation Costs
- CMS Announces Resources and Flexibilities to Assist with the Public Health Emergency in the State of Texas
- CMS Proposes New Payments for Digital Health Under CY2025 PFS Draft Rule
- Improving Public Health by Strengthening Community Infrastructure
- Biden Harris Administration Proposes Policies to Reduce Maternal Mortality, Advance Health Equity, and Support Underserved Communities
- Nearly Half of U.S. Counties Don't Have a Single Cardiologist
- Randolph County, Ill. Turns Unused Part of Nursing Home Into State-Of-The-Art Behavioral Health Center
- Safe and Stable Housing Is a Foundation of Successful Recovery
- Rural RPM Program Is a Lifeline for Pregnant Women
- Expert: Rural Hospitals Are Particularly Vulnerable to Increasing Cyberattacks Targeting Healthcare Facilities
- Biden-Harris Administration Invests Over $200 Million to Help Primary Care Doctors, Nurses, and Other Health Care Providers Improve Care for Older Adults
- AJPH Call for Papers Special Section on Intersections of Public Health And Primary Care
- NIH HEAL Initiative Turns Attention to Pragmatic Trials in Rural Communities
Unlike Previous Stimulus Package, Relief Funding Will Reach Every U.S. County

By Olivia Weeks
The American Rescue Plan will distribute more than $130 billion to local governments. Included in that total is nearly $9 billion for rural county governments, plus additional funds for the cities located in those counties.
Read more
Signing of the American Rescue Plan

The Biden-Harris Administration announced the American Rescue Plan Act of 2021 (ARP) will help to reduce health care costs, expand access to coverage, and ensure nearly everyone who buys their own individual or family health insurance through a Marketplace can receive a tax credit to reduce their premiums. The ARP not only provides the resources for America to beat this pandemic, but it also expands access to health insurance coverage, lowers costs, and ensures that health care truly is a right for all Americans.
The fact sheets cover more details on the provisions to be implemented April 1. Look for more communication from CMS over the next week for training sessions that will provide more information.
To read the CMS fact sheet, visit: https://www.cms.gov/newsroom/fact-sheets/american-rescue-plan-and-marketplace
To read the HHS fact sheet, visit: https://www.hhs.gov/about/news/2021/03/12/fact-sheet-american-rescue-plan-reduces-health-care-costs-expands-access-insurance-coverage.html
Social Media
Please help us to amplify this announcement on your social channels using this sample language:
@POTUS signed the American Rescue Plan, which expands access to health care and financial assistance, and lowers premiums. Read the full announcement here: https://www.hhs.gov/about/news/2021/03/12/fact-sheet-american-rescue-plan-reduces-health-care-costs-expands-access-insurance-coverage.html
New Covid Infections and Deaths Decline by 20% in Rural Counties

By Tim Murphy and Tim Marema
New infections have dropped by 80 percent since their peak in mid-January. The number of deaths per week has fallen by 60 percent in the past two months.
Read more
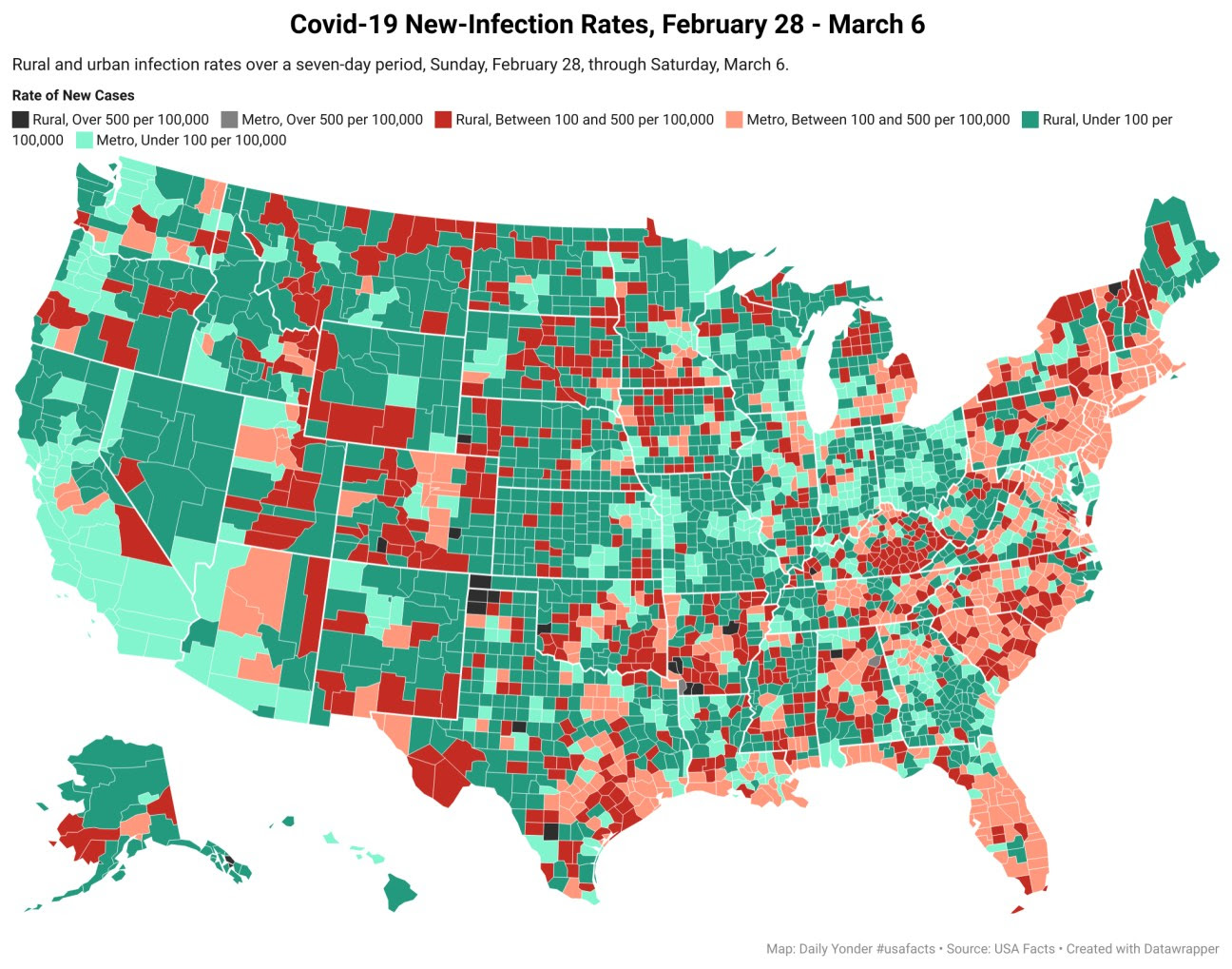
ERS on COVID-19 and Rural America
The Economic Research Service (ERS) at the U.S. Department of Agriculture analyzes county-level data on the prevalence of COVID-19 and local employment rates. A second report takes a closer look at the impact on counties where meatpacking accounts for more than 20 percent of employment.
AHRQ Data Request: Improving Rural Health Through Telehealth-Guided Provider-to-Provider Communication.
The Agency for Healthcare Research and Quality (AHRQ) is seeking published and unpublished scientific information to inform their review of this subject. Key questions in the review include those about the effectiveness of provider-to-provider telehealth and about effective implementation strategies. Submit information to AHRQ’s Evidence-Based Practice Centers Program on or before April 1.
New Public Education Videos in Spanish on COVID-19 and Flu
The Department of Health and Human Services (HHS) has published new videos in Spanish for the COVID-19 and Flu public education campaign. Share these 30- and 60-second videos in Spanish featuring Dr. Eliseo Perez-Stable, Director of the National Institute on Minority Health and Health Disparities (NIMHD) at the National Institutes of Health (NIH). ¿Son seguras para nosotros las vacunas contra el COVID? FAQ1 30- and 60-second ¿Tienen los latinos mayores de 65 años más riesgos de enfermedades graves por COVID? FAQ2 30– and 60-second.
ACS Guidebook on 2021 Messaging Now Available
The 2021 Messaging Guidebook: Effectively Messaging Cancer Screening During the COVID-19 Pandemic, which is based on American Cancer Society market research findings, is now available. The guidebook serves to assist health systems, cancer centers, healthcare providers, patient navigators, and cancer coalitions in efforts to resume preventive cancer care. The ACS is also sharing the Effectively Messaging Cancer Screening During the COVID-19 Pandemic: Issue Brief which is an abbreviated version of the guidebook. And do not forget, March is Colorectal Cancer Awareness Month!
Lessons Learned from a Year of Virtual Integrated Behavioral Health
The Health Information Technology, Evaluation and Quality Center (HITEQ) in a new resource highlights promising practices in virtual integrated behavioral health care from community health centers who rapidly transitioned to new mechanisms of care delivery through the shift to telehealth during the COVID-19 pandemic. These practices are generated from observations in group and individual conversations with health center leaders, primary care association staff, and behavioral health practitioners over a three-month period in Fall 2020. This document is intended to spark adaptation and improvement by providing a brief snapshot into the tremendous creativity and hard work that has characterized health center operations since the start of the pandemic.
At-Home Rapid Test Receives FDA Emergency Use Authorization
The Food and Drug Administration (FDA) issued an emergency use authorization on Monday for a rapid at-home COVID-19 test that delivers results without the use of an outside laboratory. The QuickVue At-Home COVID-19 Test must be obtained via prescription and can be used by individuals 14 and older, or individuals eight and older as long as an adult collects the nasal swab sample. The test can be prescribed by a healthcare provider for individuals who are within six days of symptom onset.
Hope Is on the Horizon, but Don’t Move Too Soon, Too Fast
Despite the progress, COVID-19 numbers in the U.S. are still alarmingly high. Experts are cautioning that they could quickly climb even higher if Americans let up now, and don’t retain current masking and other precautions for at least several more months, especially as the incidence of variants is on the rise. Dr. Zeke Emanuel, who was a health adviser for the Obama White House and was a member of the Biden Transition COVID-19 Advisory Board, said, “We’re still having on average 2,000 deaths a day. We cannot become inured to that.”
