- Colorectal Cancer Is Rising among Younger Adults. Some States Want to Boost Awareness.
- Rural Hospitals Built During Baby Boom Now Face Baby Bust
- Food Stamps Go Further in Rural Areas — Until You Add Transportation Costs
- CMS Announces Resources and Flexibilities to Assist with the Public Health Emergency in the State of Texas
- CMS Proposes New Payments for Digital Health Under CY2025 PFS Draft Rule
- Improving Public Health by Strengthening Community Infrastructure
- Biden Harris Administration Proposes Policies to Reduce Maternal Mortality, Advance Health Equity, and Support Underserved Communities
- Nearly Half of U.S. Counties Don't Have a Single Cardiologist
- Randolph County, Ill. Turns Unused Part of Nursing Home Into State-Of-The-Art Behavioral Health Center
- Safe and Stable Housing Is a Foundation of Successful Recovery
- Rural RPM Program Is a Lifeline for Pregnant Women
- Expert: Rural Hospitals Are Particularly Vulnerable to Increasing Cyberattacks Targeting Healthcare Facilities
- Biden-Harris Administration Invests Over $200 Million to Help Primary Care Doctors, Nurses, and Other Health Care Providers Improve Care for Older Adults
- AJPH Call for Papers Special Section on Intersections of Public Health And Primary Care
- NIH HEAL Initiative Turns Attention to Pragmatic Trials in Rural Communities
Radically Rural: Reimagining Rural Healthcare Post-Pandemic
FCC Announces Round 2 COVID-19 Telehealth Program Application Portal Will Open On April 29
Round 2 of Telehealth Program Will Provide an Additional $249 Million to Support Health Care Providers and Patients In All 50 States, DC, and Territories
The Federal Communications Commission’s Wireline Competition Bureau will begin accepting applications for Round 2 of the COVID-19 Telehealth Program on Thursday, April 29, 2021 at 12:00 PM ET at www.fcc.gov/covid19telehealth. The filing window will last seven calendar days and close on Thursday, May 6, 2021 at 12:00 PM ET. Round 2 of the COVID-19 Telehealth Program is a $249.95 million federal initiative that builds on the $200 million program established as part of the CARES Act.
“For over a year, health care providers have fought on the front lines of this pandemic and have had to rapidly innovate to support the health and well-being of all Americans. Telehealth has been at the forefront of this effort and I’m pleased to announce that additional support is just around the corner,” said Acting Chairwoman Jessica Rosenworcel. “Today the FCC announced it will open the application process for the second half of COVID-19 Telehealth Program funding later this month. The FCC is dedicated to moving quickly to review and approve applications for this funding to support health care providers and patients across the country.”
The FCC’s COVID-19 Telehealth Program supports the efforts of health care providers to continue serving their patients by providing reimbursement for telecommunications services, information services, and connected devices necessary to enable telehealth during the COVID-19 pandemic.
For additional information on eligibility and the application process, review the Application Process Guidance available on the Universal Service Administrative Company’s COVID-19 Telehealth Program webpage at https://www.usac.org/about/covid-19-telehealth-program/.
Questions specific to the application process should be directed to Round2TelehealthApplicationSupport@usac.org.
Pennsylvania Ag Secretary Hosts Virtual Discussion Encouraging Confidence in Science to Pennsylvania Ag Industry
With all Pennsylvania adults now eligible for the COVID-19 vaccine, Pennsylvania Secretary of Agriculture Russell Redding hosted a virtual discussion with Pennsylvania farmers who have already received the COVID-19 vaccine. They were joined by Dr. Mark Goedecker, regional medical director for WellSpan Health, who discussed the value of vaccinated Pennsylvanians sharing their story to boosting confidence and acceptance among others.
“This vaccine is as essential as our agriculture industry and we want to arm them with the information they need to make decisions with confidence,” said Redding. “Today we heard from farmers who shared their ‘why’ for getting vaccinated and that commonly included something more essential than food and health: family.
“We’ve all missed out over the past year, and one thing we can’t afford to lose is more time with those we love. Parents, children, brothers and sisters – there’s nothing to replace them. They are the ultimate reason.”
Those who work in Pennsylvania’s essential food and agriculture industry and choose to get the COVID-19 vaccine are protecting themselves, their family, their co-workers, and their community. In addition to this, they’re protecting the availability and accessibility of food. Vaccination is a personal decision which is highly influenced by confidence. Vaccine champions – those who have already been successfully vaccinated – are critical to building community confidence.
“At WellSpan Health we are committed to decreasing vaccine hesitancy, and it starts with educating those in our communities on the science, while also working to remove barriers to accessing the vaccine,” explained Goedecker. “We can and will overcome this pandemic, but it takes all of us doing our part to make that a reality. This shot of hope is a huge step in getting us there.”
Dr. Goedecker discussed the importance of those interested in learning about the COVID-19 vaccine to find information from credible sources that are regularly updated. And while the internet is a useful tool for research, when it comes to health-related issues the internet should not replace a discussion with a healthcare professional.
During a Facebook Live event, three Pennsylvania farmers discussed their reasons for choosing to get the COVID-19 vaccine.
Chris Hoffman, PA Farm Bureau Vice President, Mifflin & Juniata County Farmer
“I got the COVID-19 vaccine to protect my health and my family’s health,” said Hoffman. “Plus, if I do get COVID down the line, the antibodies from the vaccine will lessen the affect and decrease my risk for serious illness.
“I understand that getting the vaccine is a personal choice, but I have chosen to trust the science…just as I do on the farm. We use vaccine to protect the health and safety of our animals in our herd. If we accept science and technology in farming, we should do the same for our own health.”
John Good, The Good Farm, Lehigh County Organic Vegetable Farmer
“We operate a small family farm with a three- to five-person field-crew. One of our greatest fears over the past year was getting sick with COVID-19 and unable to work for a few weeks,” said Good. “This would be incredibly difficult for us to deal with during any season on a vegetable farm, when production schedules are always extremely tight. It could ruin our entire season. Another reason that was very important for us was to be able to spend more time with our parents, who are high-risk individuals.”
Recognizing some farmers are on the fence and leery about the process, John provided some advice and perspective.
“It’s worth it for so many reasons. The sense of relief you will feel after you get your shots and know it’s one less thing you will have to worry about in the background of an always busy farming season is probably reason enough,” said Good. “But also, the only way we are going to end this pandemic once and for all is through vaccination. We felt it was our duty as responsible citizens to be a part of that solution.
“The vaccination process was very efficient and simple. We had minor side effects like a sore arm and feeling a little under the weather for a day, but nothing too big. We are so happy to see widely available vaccinations and a decreasing level of community spread in our county,” added Good.
Phoebe Brubaker, Village Acres Farm, Juniata County Vegetable and Flower Farmer
“I couldn’t wait to get vaccinated. It gave me so much hope that we could safely return to our farmers markets and distribution sites this summer without worrying about spreading a dangerous virus to our customers,” said Brubaker. “It’s also a way for me to protect my mom, who is in her late seventies, and a very integral part of our farming operation.”
Phoebe talked about the impact of COVID-19 on rural communities. While COVID-19 hit them later than more urban areas of Pennsylvania, hospitals were quickly overwhelmed.
“We need to do our part to protect our communities and our elders,” Brubaker added. “They hold a wealth of information about farming and many have weathered the hardships of small pox and measles outbreaks. They did their part to get vaccinated then and control the disease. Now it’s our turn.”
State Resources
The Wolf Administration stresses the role Pennsylvanians play in helping to reduce the spread of COVID-19:
- Wash your hands with soap and water for at least 20 seconds or use hand sanitizer if soap and water are not available.
- Cover any coughs or sneezes with your elbow, not your hands.
- Clean surfaces frequently.
- Stay home to avoid spreading COVID-19, especially if you are unwell.
- If you must go out, you are required to wear a mask when in a business or where it is difficult to maintain proper social distancing.
- Download the COVID Alert PA app and make your phone part of the fight. The free app can be found in the Google Play Store and the Apple App Store by searching for “covid alert pa”.
Pennsylvania Leadership Encourages All College Students to Receive COVID-19 Vaccine Before the Semester Ends
The Pennsylvania Departments of Health (DOH) and Education (PDE) encouraged all students at Pennsylvania colleges and universities to receive their COVID-19 vaccination before the semester ends. DOH and PDE officials are encouraging all institutions of higher education to work with local providers to ensure vaccination opportunities are available before students leave for the summer.
“As students are now eligible for the COVID-19 vaccine, we encourage them to find a provider and get vaccinated before they travel back home at the end of the semester,” Acting Secretary of Health Alison Beam said. “We look forward to higher education institutions connecting with our provider network or the Federal Retail Pharmacy Partners to coordinate vaccination opportunities for their students.”
The DOH vaccine jurisdiction includes 66 counties across Pennsylvania, and everyone over 16 is eligible for the COVID-19 vaccine – regardless of occupation, health conditions, residency, or citizenship. Students do not have to be a resident of Pennsylvania to receive the vaccine here.
“The COVID-19 vaccine will allow postsecondary institutions to provide more in-person learning and improve the safety of our campus communities for our students, faculty, and staff,” said Acting Secretary of Education Noe Ortega. “I encourage all students enrolled in PA’s postsecondary institutions to take advantage of this available resource and get the vaccine before the semester ends.”
“There are safe and effective vaccines available, and we encourage all students to get vaccinated today. Even if students are concerned about not getting their second dose while at school, it is important to seek out the vaccine now and to later find the second dose if needed,” Acting Physician General Dr. Denise Johnson said. “When fully vaccinated, students can travel home safely knowing they are armed with the best protection against this virus. Even when vaccinated, it is important to wear a mask, practice social distancing, and wash hands frequently.”
To date, more than 6.6 million doses have been administered to more than 4.3 million people. You can find vaccine demographics in our vaccine dashboard here.
Students can visit the DOH vaccine map to find a provider nearest them or call the PA Health Hotline at 1-877-PA-HEALTH (1-877-724-3258) with questions.
While vaccine supply from the federal government remains limited, the Department of Health is working to ensure the vaccine is provided in a way that is ethical, equitable and efficient.
- The Your Turn tool provides a way to register to be alerted when it’s your turn to be vaccinated.
- A commonwealth COVID-19 vaccination guide explains the current process for getting one. Pennsylvanians with questions about the vaccination process can call the Department of Health hotline at 1-877-724-3258.
- Vaccine provider map to find a COVID-19 vaccine provider near you.
- All of the locations that received vaccine and how much they have received can be found on the COVID-19 Vaccine Distribution webpage.
- Vaccine dashboard data can also be found on the website to find more information on the doses administered and showcase demographic information.
- Pennsylvanians can provide feedback on the Pennsylvania COVID-19 Interim Vaccination Plan by clicking on the Plan Feedback Form square under Popular Vaccine Topics here.
- Frequently asked questions can be found here.
NIOSH COVID-19 Resources

As part of NIOSH’s efforts to keep our readers up to date on the CDC and NIOSH COVID-19 response, here is a summary of new information available:
Vaccination Resources
Post-vaccination Considerations for Workplaces
CDC has developed information for workplaces to help them properly evaluate and manage employees who have signs and symptoms after receiving a COVID-19 vaccine. Occupational health programs and public health officials can use the strategies, which apply to employees working in various settings.
Ventilation Resources
Upper-room Ultraviolet Germicidal Irradiation
CDC published this new webpage with information on upper-room ultraviolent germicidal irradiation (UVGI), which can be effective at reducing exposures to SARS-CoV-2 in some group settings. SARS-CoV-2 is the virus that causes COVID-19. UVGI uses ultraviolet (UV) energy to kill viral, bacterial, and fungal organisms. Other ventilation strategies, such as fans that bring in outdoor air, high-efficiency particulate air (HEPA) filter systems, and open windows, can also help reduce infectious viral particles in the air.
For More Information
For more information, please visit the COVID-19 webpage. To stay up to date on new developments, sign up for the COVID-19 newsletter.
CMS: Vaccine Resources: 4/16/21 – J&J Vaccine Update and HHS Key Messages

As COVID-19 vaccines continue rolling out across the country, CMS is taking action to protect the health and safety of our nation’s patients and providers and keeping you updated on the latest COVID-19 resources from HHS, CDC and CMS.
With information coming from many different sources, CMS has up-to-date resources and materials to help you share important and relevant information on the COVID-19 vaccine with the people that you serve. You can find these and more resources on the COVID-19 Partner Resources Page and the HHS COVID Education Campaign page. We look forward to partnering with you to encourage our beneficiaries to get vaccinated when they have the opportunity. For more information, visit the CMS COVID-19 Policies and Guidance page.
Information for Providers
J&J COVID-19 Vaccine: Health Alert
The CDC issued a Health Alert, about the CDC and FDA’s recommended pause in the use of the J&J COVID-19 vaccine, in part, to ensure that the health care provider community is aware of the potential for adverse events and can provide proper management due to the unique treatment required with this type of blood clot. This alert includes specific recommendations for clinicians.
Information for Partners
A message from the COVID-19 Community Corps::
Tuesday night, you and nearly 2,500 fellow trusted messengers joined Dr. Fauci and me to discuss the Johnson & Johnson (J&J) vaccine recommended pause. Thank you for being there. Your leadership in sharing the latest information about COVID-19 vaccines with the communities you serve and engage is essential to addressing this pandemic. I’d like to take a moment to summarize our discussion:
- On Tuesday (4/13), the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA) announced they are reviewing data involving a small number of reported cases of a rare and serious type of blood clot in individuals after receiving the J&J vaccine. FDA and CDC, out of an abundance of caution, recommended a pause in the use of the J&J vaccine as they review this data.
- Based on what we know now, these blood clots are extremely rare. At the time of the announcement, a small number of cases (6) were reported out of the nearly seven (7) million doses of the J&J vaccine administered so far in the United States.
- If you received the J&J vaccine more than three weeks ago, your risk of developing a blood clot is very low. If you got this vaccine within the last three weeks, your risk of developing a blood clot is also very low. That said, you should be on the lookout for possible symptoms of a clot, which the CDC describes, here.
- The news about the J&J vaccine pause does not affect the two other vaccines that are widely used in the United States – Pfizer and Moderna. More than 100 million people in the U.S. have been vaccinated safely with these vaccines over the past several months.
- We are still confident in the overall supply of COVID-19 vaccines for the country. The Administration has secured enough Pfizer and Moderna doses for 300 million Americans and there is more than enough supply to continue the current pace of vaccinations of three (3) million shots per day.
- For people who already have appointments for J&J vaccines, state and federal partners are working to get these appointments rescheduled for a Pfizer or Moderna vaccine.
- The decision to recommend a pause in administration of the J&J vaccine shows the rigorous steps that the FDA are taking to ensure that the American people have clear and transparent information about the safety and effectiveness of these vaccines. Americans should be confident that even when the occurrence of side effects are extremely rare, as is the case here, the CDC and FDA will take every necessary step to communicate those to the public.
- Yesterday (4/14), the Advisory Committee on Immunization Practices (ACIP) held a public meeting to review in detail the information we have so far, which you can watch here. The Committee will reconvene as quickly as possible in the next two weeks to review any additional scientific evidence and deliberate further. CDC and FDA will carefully consider the Committee’s recommendations when they are made. I appreciate ACIP convening quickly and experts providing advice that prioritizes safety.
- Here’s the bottom line: The COVID-19 vaccines have already saved lives, and we still have vaccine options that are safe and effective, and Americans should continue to get vaccinated as soon as possible.
- As a resource, more information about the safety of COVID-19 vaccines can always be found here.
I invite you to watch and share these resources with your community:
- SHARE a message I posted last night, here.
- SHARE Dr. Fauci’s video on what people need to know about the J&J vaccine recommended pause. ( Facebook, Twitter, Instagram, and YouTube).
- SHARE a new Q&A released from the CDC, here.
- SHARE and LISTEN to a replay of FDA/CDC Joint Media Call on the FDA YouTube channel.
- SHARE and FEATURE our COVID-19 Community Corps Members that tweeted about the event yesterday: @Rikoamour, @SheilaKatz1, @_EricCarr, @bhrenton, @mjaeckel
Thank you for your continued partnership in protecting the health of our nation.
Dr. Vivek Murthy
Surgeon General of the United States
Vaccine Hesitancy for COVID-19: State, County, and Local Estimates
To support state and local outreach efforts, HHS researched federal survey data to predict vaccine hesitancy rates down to the county level. There are two Excel spreadsheets: one presents state and county estimates of the proportion of adults who describe themselves as “probably not” (Hesitant) or “definitely not” (Strongly Hesitant) going to get a COVID-19 vaccine once one is available to them, and the other presents select sociodemographic and geographic factors by county that can be examined together with the estimates of vaccine hesitancy. See the map here.
Covid Racial Disparities Loom Large in Rural Counties

By Aallyah Wright, Stateline
Rural counties in states that failed to expand Medicaid see disparities in access to Covid vaccines along the racial lines grow even bigger.
Read more
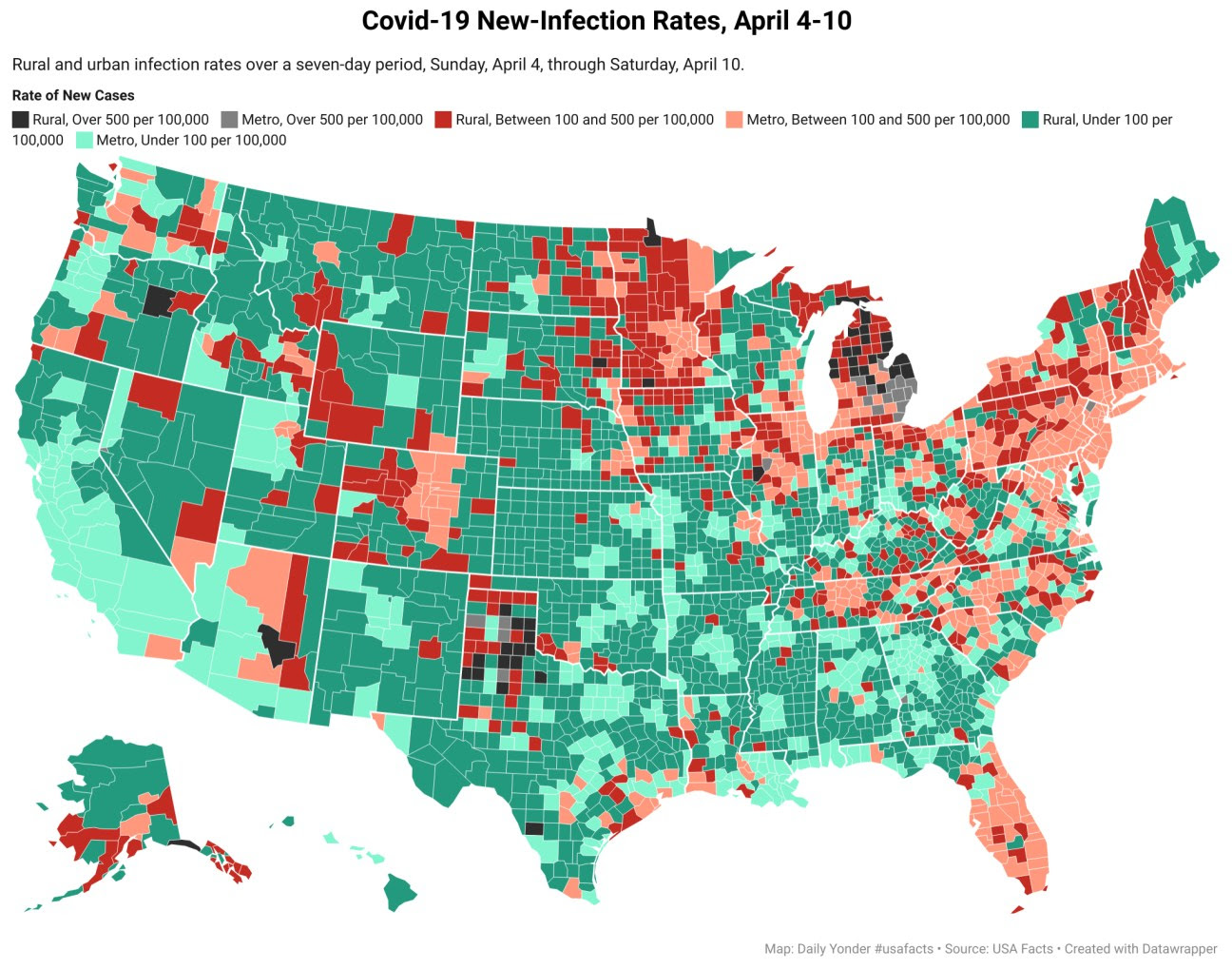
New Covid Cases Increase 10% in Rural Counties

By Tim Murphy and Tim Marema
Michigan and northwest Texas are the hottest spots in the spread of Covid-19 in rural areas.
Read more
FDA Fact Sheets Address Variants for Monoclonal Antibody Products
The U.S. Food and Drug Administration (FDA) recently released revised fact sheets for health care providers that include additional information on susceptibility of SARS-CoV-2 variants to each monoclonal antibody therapy available through an Emergency Use Authorization for COVID-19 treatment. The fact sheets contain details regarding specific variants and potential resistance. Download revised fact sheets for: Bamlanivimab; Bamlanivimab and Etesevimab and REGEN-COV™ (Casirivimab with Imdevimab).

