- Colorectal Cancer Is Rising among Younger Adults. Some States Want to Boost Awareness.
- Rural Hospitals Built During Baby Boom Now Face Baby Bust
- Food Stamps Go Further in Rural Areas — Until You Add Transportation Costs
- CMS Announces Resources and Flexibilities to Assist with the Public Health Emergency in the State of Texas
- CMS Proposes New Payments for Digital Health Under CY2025 PFS Draft Rule
- Improving Public Health by Strengthening Community Infrastructure
- Biden Harris Administration Proposes Policies to Reduce Maternal Mortality, Advance Health Equity, and Support Underserved Communities
- Nearly Half of U.S. Counties Don't Have a Single Cardiologist
- Randolph County, Ill. Turns Unused Part of Nursing Home Into State-Of-The-Art Behavioral Health Center
- Safe and Stable Housing Is a Foundation of Successful Recovery
- Rural RPM Program Is a Lifeline for Pregnant Women
- Expert: Rural Hospitals Are Particularly Vulnerable to Increasing Cyberattacks Targeting Healthcare Facilities
- Biden-Harris Administration Invests Over $200 Million to Help Primary Care Doctors, Nurses, and Other Health Care Providers Improve Care for Older Adults
- AJPH Call for Papers Special Section on Intersections of Public Health And Primary Care
- NIH HEAL Initiative Turns Attention to Pragmatic Trials in Rural Communities
CMS Data Shows Vulnerable Americans Forgoing Mental Health Care During COVID-19 Pandemic

Findings from New Medicaid & CHIP Data Analysis Correspond with Reports of Adverse Mental Health Conditions for Vulnerable Populations
The Centers for Medicare & Medicaid Services (CMS) released data today highlighting the continued impact the COVID-19 Public Health Emergency (PHE) is having on Medicaid and Children’s Health Insurance Program (CHIP) beneficiaries and utilization of health services. The data show that, from March through October 2020, beneficiaries have foregone millions of primary, preventive, and mental health care visits due to the COVID-19 PHE, compared to the same time period in 2019. Although utilization rates for some treatments have rebounded to pre-pandemic levels, mental health services show the slowest rebound.
This decline in utilization is occurring at a time when preliminary evidence shows mental health conditions have worsened nationwide. The gap in service utilization due to the PHE, particularly for mental health services, may have a substantial impact on long-term health outcomes. Medicaid and CHIP-funded mental health services, in addition to primary and preventative services, cover the majority of children, people living in poverty, and those with special health care needs. Medicaid and CHIP also cover millions of racial and ethnic minorities.
To help close this gap in services, CMS is emphasizing mental health care in its recently launched Connecting Kids to Coverage National Campaign, a national outreach and enrollment initiative funded under the Children’s Health Insurance Program Reauthorization Act (CHIPRA) and the Affordable Care Act, that reaches out to families with children and teens eligible for Medicaid and CHIP.
The Biden-Harris Administration is committed to achieving mental health parity, expanding access to mental health care, and eliminating mental health stigmas. In addition to improving access to mental health services provided to Medicaid and CHIP beneficiaries, President Biden’s American Rescue Plan provided funding to addresses our mental health and substance use challenges, including $3 billion in funding for block grants to address mental health and substance use prevention, treatment, and recovery services. This is alongside $1.4 billion to support the mental health needs of health care professionals and first responders, as well as funding specifically for pediatric mental health.
“More than 100 million Americans, including 43 million children, relied on us to deliver access to mental health and other services they needed through Medicaid and CHIP in 2020. Medicaid is the largest payer in the nation for mental health and that care is a lifeline for many,” said CMS Acting Administrator Liz Richter. “This new data provides a window into the impacts of the pandemic for marginalized communities – particularly children and other vulnerable people – and is critical as we work towards meeting the needs of those that rely on Medicaid and CHIP. While we’re encouraged that people are accessing some health care services at pre-pandemic levels, there is work to do to connect people to mental health care services and to ensure we fill the gap in other types of services that was caused by the pandemic. The Centers for Medicare & Medicaid Services is committed to connecting people and children to health care – including mental health care.”
Specifically, the results demonstrate a 34% decline in the number of mental health services utilized by children under age 19, compared to the same time period in 2019, and 22% decline in the number of mental health services utilized by adults aged 19 to 64, compared to the same time period in 2019. This translates to approximately 14 million fewer mental health services for children and approximately 12 million fewer mental health services for adults, for a total of nearly 26 million fewer mental health services utilized across both groups. Similarly, although there are preliminary reports of increased drug-related mortality due to the COVID-19 PHE, substance use disorder service utilization fell by 3.6 million services (13% decline) when compared to the same time period in 2019.
It is important to note that the data does show that utilization rates for certain primary and preventive services for children under age 19 have recovered to pre-pandemic levels, or have started to rebound across many areas of the country. While this recovery is encouraging, millions of services still need to be delivered to make up for those missed between March and October 2020. Preliminary 2020 data shows 9% fewer childhood vaccinations for beneficiaries under age two (1.8 million services), 21% fewer child screening services (4.6 million services) among children under age 19, and 39% fewer dental services (11.4 million services) among children under age 19 when compared to pre-pandemic levels. This data takes into account increases in telehealth utilization of services via telehealth.
Throughout the pandemic, CMS has encouraged states to consider telehealth options to combat COVID-19 and increase access to care. This updated data snapshot demonstrates a marked increase in the number of services delivered via telehealth compared to prior years. The number of services delivered via telehealth surged 2,700% during the PHE to nearly 68 million between March and October 2020. However, this increase has not been enough to offset the decline in service utilization in other areas.
For COVID-19 treatment and acute care use, the preliminary findings show more than 1.2 million Medicaid and CHIP beneficiaries received COVID-19 treatment, and nearly 124,000 were hospitalized through October 2020. Despite significant variance across states regarding this data, preliminary results suggest that the COVID-19 treatment rate increases with age. In addition, Medicaid and CHIP paid for nearly 10 million COVID-19 tests or testing related services, although this data does not include tests provided free of charge or covered by other insurance programs, including Medicare.
CMS will continue monitoring and working with states, providers and stakeholders to develop and implement innovative ways to provide access to critical health care such as preventive childhood vaccinations and mental health services to beneficiaries enrolled in Medicaid and CHIP.
The data released today can be found here: https://medicaid.gov/state-resource-center/downloads/covid-19-medicaid-data-snapshot.pdf
For a fact sheet on the Medicaid & CHIP and the COVID-19 Public Health Emergency, please visit: https://www.cms.gov/newsroom/fact-sheets/fact-sheet-medicaid-chip-and-covid-19-public-health-emergency
CMS Builds on Whole-of-Government COVID-19 Response with Vaccination Education, Offering, and Reporting

As part of the ongoing response to address the COVID-19 pandemic and to improve health care access and reduce the risk of severe illness and death from COVID-19, CMS issued a rule that will ensure long-term care facilities, and residential facilities serving clients with intellectual disabilities, educate and offer the COVID-19 vaccine to residents, clients, and staff. These requirements apply to Long-Term Care (LTC) facilities and Intermediate Care Facilities for Individuals with Intellectual Disabilities (ICFs-IID) and align with existing requirements for influenza and pneumococcal vaccines in LTC facilities.
The rule also requires LTC facilities to report weekly COVID-19 vaccination status data for both residents and staff. The new vaccination reporting requirement will not only assist in monitoring uptake amongst residents and staff but will also aid in identifying facilities that may be in need of additional resources and/or assistance to respond to the COVID-19 pandemic.
“These new requirements reinforce CMS’ commitment of ensuring equitable vaccine access for Medicare and Medicaid beneficiaries,” said Dr. Lee Fleisher, MD, CMS Chief Medical Officer and Director of CMS’ Center for Clinical Standards and Quality (CCSQ). “Today’s announcement directly aids nursing home residents and people with intellectual or developmental disabilities who have been disproportionately affected by COVID-19. Our goal is to increase COVID-19 vaccine confidence and acceptance among these individuals and the staff who serve them.”
To ensure LTC facilities receive support for COVID-19 vaccination efforts, they are now required to report weekly vaccination data of residents and staff to the CDC National Healthcare Safety Network (NHSN), the nation’s most widely used health care-associated infection tracking system. LTC facilities are already required to report COVID-19 testing, case, and mortality data to the NHSN for residents and staff but have not been required to report vaccination data. As data becomes available, CMS will post facility-specific vaccination status information reported to the NHSN for viewing by facilities, stakeholders, and the public on CMS’ COVID-19 Nursing Home Data website.
While this announcement is specific to LTC facilities and ICFs-IID, CMS is also seeking comment on opportunities to expand these policies to help encourage vaccine uptake and access in other congregate care settings, such as psychiatric residential treatment facilities, group homes, and assisted living facilities. By requiring vaccine education and offering within LTC facilities and ICFs-IIDs, CMS is improving health care access and reducing the risk of severe illness and death from COVID-19.
More Information:
Coming Soon – New HHS funding for Rural Health Clinics to Strengthen COVID-19 Testing and Mitigation, Increase Vaccine Confidence
The Health Resources and Services Administration’s (HRSA) FORHP will be making new U.S. Department of Health and Human Services (HHS) funding available for Rural Health Clinics (RHCs) to strengthen COVID-19 testing and mitigation and increase vaccine confidence.
Rural Health Clinic Vaccine Confidence (RHCVC) Program – Coming Soon!
Interested RHCs should watch for the upcoming funding opportunity and start the process to register to apply for a HRSA grant. HRSA will fund all eligible RHCs that have a complete and acceptable application. RHCs may use this funding to increase vaccine confidence, improve health care in rural areas, and reinforce key messages about prevention and treatment of COVID-19 and other infectious diseases.
For additional information please register in advance and join the National Association of Rural Health Clinic’s (NARHC) RHC COVID Initiatives webinar on Wednesday May 19th, at 12 PM ET. Please join the RHC Vaccine Confidence Program mailing list for additional program announcements and updates by clicking here. For additional questions: RHCVaxConfidence@hrsa.gov.
Rural Health Clinic COVID-19 Testing and Mitigation Program – Coming Soon!
RHCs that have met the requirements for the RHC COVID-19 Testing Program will receive a one-time allocation of up to $100,000 per clinic site automatically deposited in the bank account of the corresponding TIN organization in June 2021. RHCs will not have to apply for payments for this program. Ineligible RHCs that are not current with reporting of testing data on rhccovid19reporting.com will have the opportunity to catch up for future program consideration, dependent on the availability of funds. Please join the RHC COVID-19 Testing Program mailing list for additional program announcements and updates by clicking here. For additional questions: RHCCOVID-19Testing@hrsa.gov.
CMS Increases Medicare Payment for COVID-19 Monoclonal Antibody Infusions

New payment policy for at-home administration
As part of the ongoing response to address the COVID-19 pandemic, the Centers for Medicare & Medicaid Services (CMS) has increased the Medicare payment rate for administering monoclonal antibodies to treat beneficiaries with COVID-19, continuing coverage under the Medicare Part B COVID-19 vaccine benefit. Beneficiaries pay nothing out of pocket, regardless of where the service is furnished – including in a physician’s office, healthcare facility or at home.
The national average payment rate will increase from $310 to $450 for most health care settings. In support of providers’ efforts to prevent the spread of COVID-19, CMS will also establish a higher national payment rate of $750 when monoclonal antibodies are administered in the beneficiary’s home, including the beneficiary’s permanent residence or temporary lodging (e.g., hotel/motel, cruise ship, hostel, or homeless shelter).
The new national payment rate for at-home administration of monoclonal antibodies accounts for increased costs associated with the one-on-one nature of this care model. These higher national average payment rates reflect additional information provided to CMS about the costs of providing these services in a safe and timely manner, such as clinical staff and personal protective equipment. This action also means Medicare payments to providers and suppliers will be more aligned to their costs to administer these products.
CMS’s goal during the COVID-19 PHE has been to ensure that the agency is supporting beneficiary access to care. This new policy is based on timely, valuable input from stakeholders including the home health and ambulatory infusion industries on the costs associated with administering monoclonal antibodies.
CMS is updating the set of toolkits for providers, states and insurers to help the health care system swiftly administer monoclonal antibody treatment with these new Medicare payment rates, at https://www.cms.gov/medicare/covid-19/monoclonal-antibody-covid-19-infusion. In addition, CMS is updating coding resources for providers, at https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/covid-19-vaccines-and-monoclonal-antibodies.
For additional clinical information about COVID-19 monoclonal antibodies, please visit:
COVID-19 Vaccine Resources: What Partners Need to Know Now 5/10/2021

As COVID-19 vaccines continue rolling out across the country, CMS is taking action to protect the health and safety of our nation’s patients and providers and keeping you updated on the latest COVID-19 resources from the Department of Health and Human Services (HHS), the Centers for Disease Control and Prevention (CDC) and the Centers for Medicare & Medicaid Services (CMS).
With information coming from many different sources, CMS has up-to-date resources and materials to help you share important and relevant information on the COVID-19 vaccine with the people that you serve. You can find these and more resources on the COVID-19 Partner Resources Page and the HHS COVID Education Campaign page. We look forward to partnering with you to encourage our beneficiaries to get vaccinated when they have the opportunity. For more information, visit the CMS COVID-19 Policies and Guidance page.
VACCINES.GOV MAKING IT EASIER TO FIND VACCINES
Access to a vaccine should not be an obstacle for someone to get vaccinated. Here are three vaccine tools to bring to your communities right now:
- Visit vaccines.gov (English) or vacunas.gov (Spanish) to search and find a vaccine near you.
- Text GETVAX (438829) for English or VACUNA (822862) for Spanish to receive three vaccine sites on your phone within seconds.
- Call the National COVID-19 Vaccination Assistance Hotline at 1-800-232-0233 for those who prefer to get information via phone call.
NEW COVID-19 RESOURCES: SPREAD THE WORD
COVID-19 Community Champions
On May 5, 2021, CMS debuted the first social media videos highlighting long-term care staff, also referred to as Community Champions, who moved from being initially uncertain about receiving the COVID-19 vaccine to accepting the vaccine– and encouraging their peers to do the same.
Throughout the COVID-19 pandemic, staff in nursing homes have been providing ongoing care to our nation’s most vulnerable. This social media campaign is intended to help increase vaccine acceptance amongst long-term care staff. Please like and share our Community Champions video: https://youtu.be/k0WbAhveyDY We can do this!
COVID-19 conference cards
Conference cards are available to order from the CMS Product Ordering web site in multiple languages. They can be found under the Featured Medicare button:
- Bring Your Medicare Card When You Get Your Covid-19 Vaccine
- Stay Protected from Covid-19 – Medicare Covers the Vaccine
Other Medicare publications are available to download in several languages here. You can also find helpful tips on CMS product ordering here.
COVID-19-RELATED COVERAGE AND PAYMENT
HRSA COVID-19 Coverage Assistance Fund (CAF)
On May 3, 2021, HHS, through the Health Resources and Services Administration (HRSA), announced a new program covering the cost of administering COVID-19 vaccines to patients enrolled in health plans that either do not cover vaccination fees or cover them with patient cost-sharing. This new program is called the HRSA COVID-19 Coverage Assistance Fund (CAF).
“After securing enough COVID-19 vaccines for all adults, the Biden-Harris Administration is elevating work to boost access to them,” said HHS Secretary Becerra. “We listened to our healthcare providers on the frontlines of the pandemic. On top of increasing reimbursement rates tied to administering the shots, we are closing the final payment gap that resulted as vaccines were administered to underinsured individuals. No healthcare provider should hesitate to deliver these critical vaccines to patients over reimbursement cost concerns.”
See the press release on this announcement here.
Learn more about CAF here. Also, see the CAF Fact Sheet and Frequently Asked Questions about the program.
Increased Medicare Payment for Administering Monoclonal Antibodies
CMS has increased Medicare payment for administering monoclonal antibodies to treat beneficiaries with COVID-19, continuing coverage under the Medicare Part B COVID-19 Vaccine Benefit. This means more providers and suppliers are readily able to administer these treatments. Beneficiaries are not responsible for any cost sharing, regardless of where the service is furnished – including in a physician’s office, other healthcare facility or at home.
The national average payment rate has increased from $310 to $450 for most health care providers. In support of providers’ efforts to prevent the spread of COVID-19, CMS will also establish a higher national payment rate of $750 for at-home monoclonal antibodies treatment.
See updated toolkits for providers, states and insurers to help the health care system swiftly administer monoclonal antibody treatment with these new Medicare payment rates, here.
In addition, CMS is updating coding resources for providers. More information can be found here.
For more information, visit www.cms.gov/covidvax.
New Funding for Rural Clinics and Hospitals
On May 4, HHS and HRSA announced new funding thanks to the American Rescue Plan to combat COVID-19 across the country. Rural clinics and hospitals will receive nearly $1 billion dollars to strengthen COVID-19 response efforts and increase vaccinations in rural communities, and approximately $250 million will be awarded to develop and support a community-based workforce who will serve as trusted voices sharing information about vaccines, work to increase COVID-19 vaccine confidence, and address any barriers to vaccination for individuals living in vulnerable and medically underserved communities.
The HHS press release on rural clinics and hospitals funding can be found here. Visit: https://www.hhs.gov/about/news/2021/05/04/hhs-announces-nearly-1-billion-from-american-rescue-plan-for-rural-covid-19-response.html
The HHS press release on community-based workforce funding can be found here.
STAY CONNECTED
Join the We Can Do This Community Corps: Help in the fight against COVID-19 by encouraging family, friends, and your community to get vaccinated. Join the Community Corps to get tips, tools and resources to share. See https://wecandothis.hhs.gov/covidcommunitycorps
For more information, please contact us: Partnership@cms.hhs.gov
How to Talk About COVID-19 Vaccines with Friends and Family

COVID-19 vaccines are new, and it’s normal for people to have questions about them. The sheer amount of information—and misinformation—about COVID-19 vaccines can be overwhelming to anyone. You can help by listening without judgement and identifying the root of their concerns. Acknowledge their emotions so they know they have been heard. Ask open-ended questions to explore their concerns, ask permission to share information, and help them find their own reason to get vaccinated.
Click here for more information and resources.
Vaccines.gov Website Is Now Live

CMS would like to make you aware that the federally supported website that makes it easier for individuals to access COVID-19 vaccines is now live. Vaccines.gov – powered by the trusted VaccineFinder brand – is available in English and Spanish, with high accessibility standard, and will help connect Americans with locations offering vaccines near them. In addition to the website, people in the U.S. are also now able to utilize a text message service, available in both English and Spanish. People can text their ZIP code to 438829 (GETVAX) and 822862 (VACUNA) to find three locations nearby that have vaccines available.
Vaccines.gov is meant to complement the number of state and pharmacy websites that have been successfully connecting many Americans with vaccinations, by providing a unified federal resource for Americans to use no matter where they are.
In addition to the website and text messaging service, the National COVID-19 Vaccination Assistance Hotline is now available to help those who prefer to get information by phone on where to get a vaccine. Call 1-800-232-0233 to find a location near you.
Report: Broadband Became “Rural America’s Critical Connection” During Pandemic

Research from the Foundation for Rural Service underscores the transformative role broadband played in rural America during the Pandemic and looks into increasing demand for what has become a fundamental service.
by Stephen V. Smith,
The optimism that naturally accompanies the start of a new decade was met with a series of challenges as 2020 unfolded. The coronavirus pandemic was chief among these, creating a public health crisis, economic hardship, and social unrest.
Broadband was the technology that ran like a thread through efforts to combat these challenges.
A new white paper published by the Foundation for Rural Service, “Broadband Today: Rural America’s Critical Connection,” examined the role of broadband in responding to the pandemic.
“Never before has reliable access to high-speed internet been as important as it has been during the Covid-19 pandemic,” said FRS Executive Director Pam Becker.“Broadband connects us to our work, education, health care, government programs, and — most importantly — one another. This report examines the necessary benefits broadband access has provided to rural Americans throughout the pandemic and what more can be done to ensure sustainable, affordable networks for all going forward.”
The report found a 40% uptick in broadband usage between the end of 2019 and 2020. According to the research, rural networks were able to function well due to investments thanks to recent investments by local providers and an increase in the “fiber-to-the-home penetration.”
The report estimated that around 30% of the modern workforce could be working from home multiple days a week by as soon as the end of 2021, creating a permanent demand for higher speeds and upload capacity.
Health Care Providers Try Novel Ways to Get Shots in Arms of Rural Residents

by Liz Carey,
Health care providers and the Centers for Disease Control and Prevention (CDC) are trying novel ways to get as many residents vaccinated as possible.
As part of that effort, the CDC recently reached out to extension agencies and other trusted rural community resources to get factual vaccination information into communities.
Called the Extension Collaborative on Immunization Teaching and Engagement (EXCITE), the program provides extension agencies with grants to promote Covid-19 vaccination information.
“The overall goal of this great collaborative effort is to create a precision immunization program based on needs assessments and local partnerships, and then to provide immunization education and linkages to immunization opportunities for the most socially vulnerable audiences,” said Alexi Piasecki, with the CDC Vaccine Task Force. “This will be done through building trust, empowering health care personnel and engaging with communities and individuals.”
The five-year, $9.9 million program is a collaborative effort between the U.S. Department of Agriculture National Institute of Food and Agriculture and the CDC and uses the Cooperative Extension System to reach out to rural and medically underserved communities.
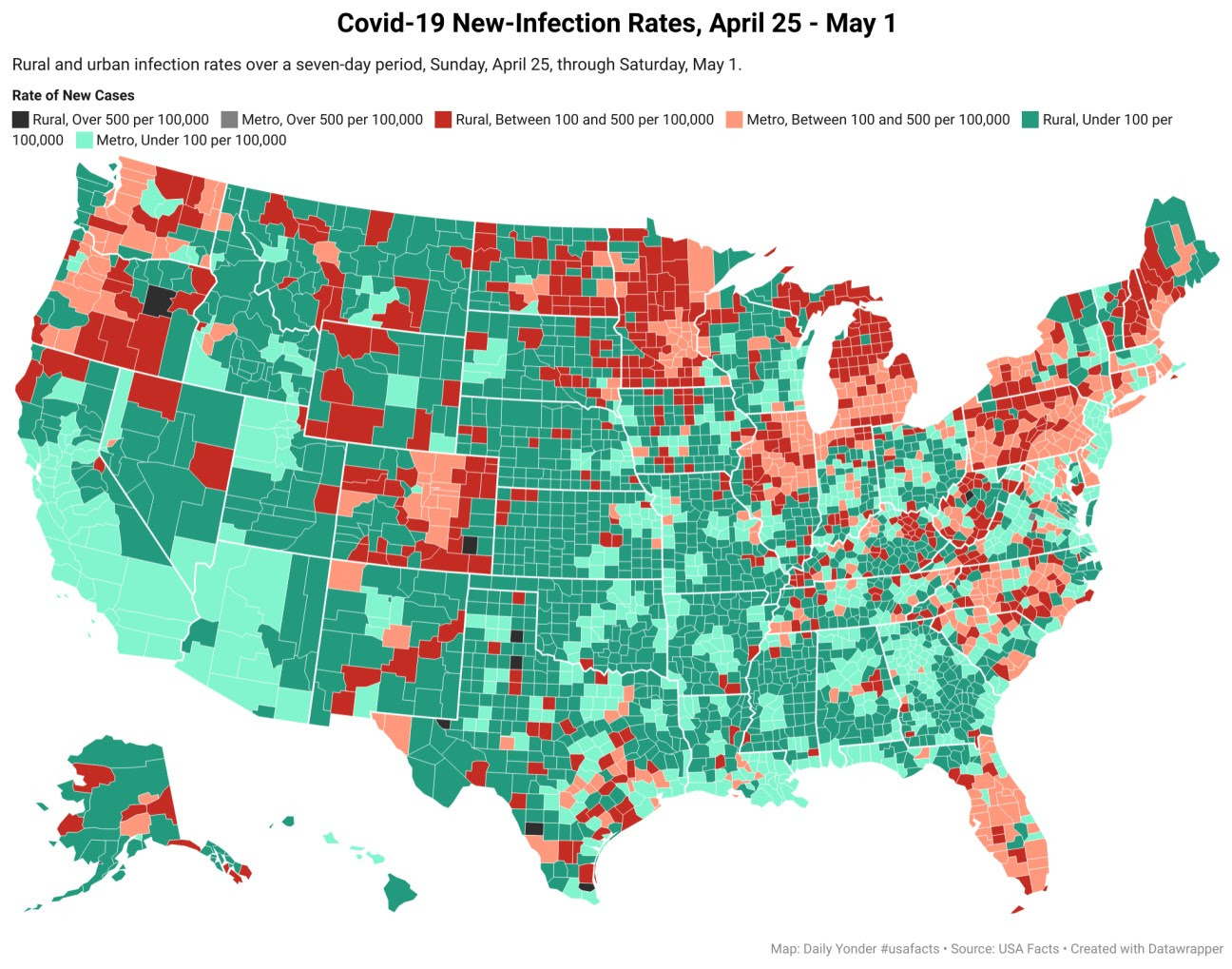
New Covid-19 Cases Drop by 5% in Rural Counties While Urban Areas See a 15% Drop

By Tim Murphy and Tim Marema
The number of new Covid-related deaths increased last week by 14%. Urban areas saw an increase of only 1%.
Read more